A Better Way To Make Biodiesel
They’re only 250 billionths of a meter in
diameter. But fill them with the right chemistry and Iowa State scientists say
the tiny nanospheres they’ve developed could revolutionize how biodiesel
is produced.
They’re only 250 billionths of a meter in diameter. But fill them with the right chemistry and Iowa State scientists say the tiny nanospheres they’ve developed could revolutionize how biodiesel is produced.
The researchers are after a new, high-tech catalyst that takes some of the energy, labor and toxic chemicals out of biodiesel production. They’ve come up with a technology that works in the laboratory. And now they’re working with the West Central Cooperative in Ralston to test their discoveries on a larger scale. They’re also working to establish a company that would move the new technology into biorefineries.
Current biodiesel production technology reacts soy oil with methanol using toxic, corrosive and flammable sodium methoxide as a catalyst. Getting biodiesel out of the chemical mixture requires acid neutralization, water washes and separation steps. It’s a tedious process that dissolves the catalysts so they can’t be used again…
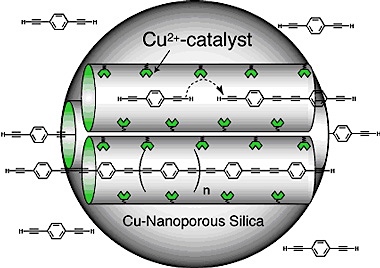
[Iowa State Professor] Victor Lin has developed a nanotechnology that accurately controls the production of tiny, uniformly shaped silica particles. Running all the way through the particles are honeycombs of relatively large channels that can be filled with a catalyst that reacts with soybean oil to create biodiesel. The particles can also be loaded with chemical gatekeepers that encourage the soybean oil to enter the channels where chemical reactions take place. The results include faster conversion to biodiesel, a catalyst that can be recycled and elimination of the wash step in the production process.
Lin’s particles can also be used as a catalyst to efficiently convert animal fats into biodiesel by creating a mixed oxide catalyst that has both acidic and basic catalytic sites. Acidic catalysts on the particle can convert the free fatty acids to biodiesel while basic catalysts can convert the oils into fuel.
And the particles themselves are environmentally safe because they are made of calcium and sand.
The process of scaling up these results to commercial levels is under way. It can’t arrive soon enough. Demand for products like this is ramping up so quickly that E85 and Biodiesel retail in my neck of the woods with a 30¢ a gallon premium added in.
Posted: Thu - June 22, 2006 at 06:26 AM